Chemical Substance Registration
According to the new and existing chemical substance registration of Executive Yuan Environmental Protection Administraion (EPA) came into force from 2014.12.11 and the New Chemical Substances registration of Ministry of Labor (MOL) came into force from 2015.01.01, we apply for the application of various types of chemical substances login service in Taiwan as the Third Party Representative (TPR) by the overseas manufacturer and domestic importers of their original commission.
Upon completion of compliance with Taiwan regulations and smooth that you can import the product to Taiwan continually, to give priority to customers' products and maintain the value of your economic interests and commercial markets, while stabilizing expanding customer base. Our responsibility is to meet your situation and needs, providing technical advice and support to meet the EPA and the MOL legislation and through the approval, the completion of Taiwan chemicals login / register job.
DG’s service items:
New Chemical Substance Registration
- Standard Registration, NST
- Simplified Registration, NSP
- Small Quantity Registration, NSQ
- Polymer of low concern (PLC) Advanced confirmation
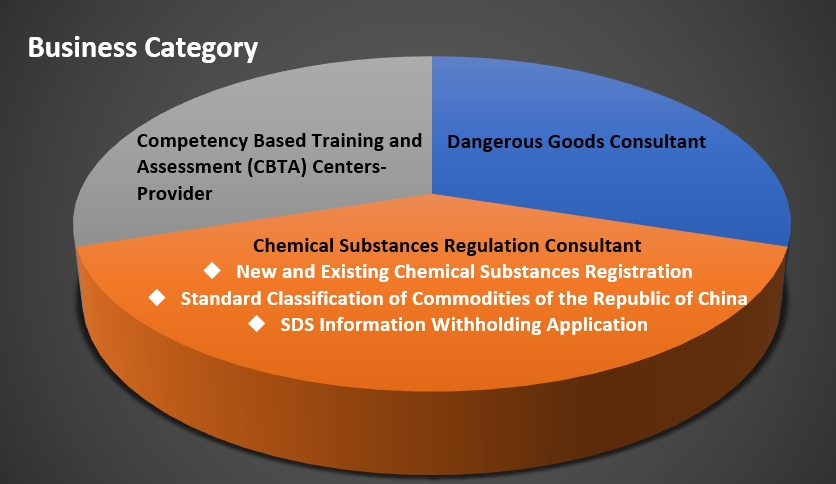
The new chemical substance registration is divided into three types basis on annual quantities of manufacture and import: NSQ, NSP and NST. The larger the annual quantities of manufacture and import, the more information should be provided. Among them, the information content of NSQ is the least, and the content of NST is the most. After the above three types of registration are approved, a registration code can be obtained from the authority, and the product can be manufactured or imported legally.
The low-concern polymers with lower hazards will be obtained pre-approval code after approval.
Legal basis:
According to Toxic and Concerned Chemical Substances Control Act, TCCSCA, Chapter 4 Registration and Reporting of Chemical Substances, Article 30. Those that manufacture or import certain quantities of existing chemical substances each year shall apply to register chemical substance data from the competent authority before the specified deadline. Those enterprises of manufacturing or importing new chemical substances shall apply to register chemical substance data from the competent authority 90 days prior to the aforementioned activities. The registered content of chemical substance data includes status of manufacture or import, physical, and chemical, toxicological, exposure characteristics, as well as hazardous assessment, and other data items designated to be included in registration by the central competent authority. Based on the annual quantities of manufacture and import along with the substance type, registration may be divided into NST, NSP and NSQ.
The branched law and order as "new chemical substances and chemical registration application "According to Regulations on New Chemical Substances Registration in Article 13, the branched law and order as "new chemical substances registration application":
Manufacturers or importers shall not manufacture or import chemicals containing new chemical substances that are not on the inventory of chemical substances announced by the central competent authority prior to submitting a chemical substance safety assessment report to the central competent authority and receiving registration approval for the new substances. Substances stipulated by other legislations or which are announced by the central competent authority announces as not applicable shall not be subject to this restriction. In order to prevent hazards to the safety and health of workers, the assessment reports in the preceding paragraph may be made public by the central competent authority after examination. Regulations governing the announcement of the inventory of chemical substances, registration of new chemical substances, content of assessment reports, examination procedures, and public disclosure of information in the preceding two paragraphs and other binding matters shall be stipulated by the central competent authority.
Registration types for new chemical substance registration:
.jpg)
Existing Chemical Substance Registration
Legal basis:
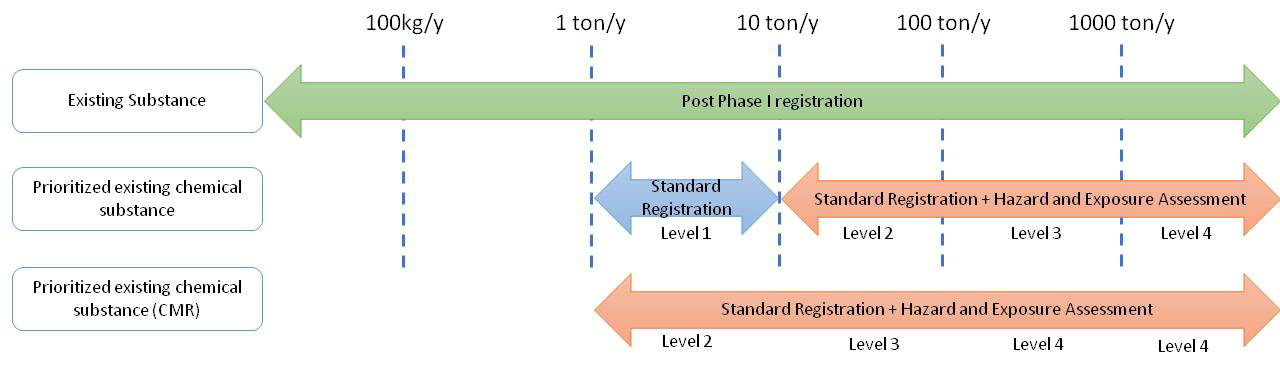
According to Toxic and Concerned Chemical Substances Control Act, TCCSCA, Chapter 4 Registration and Reporting of Chemical Substances, Article 30. Those that manufacture or import certain quantities of existed chemical substances each year shall apply to register chemical substance data from the competent authority before the specified deadline. Those enterprises of manufacturing or importing new chemical substances shall apply to register chemical substance data from the competent authority 90 days prior to the aforementioned activities. The registered content of chemical substance data includes status of manufacture or import, physical, and chemical, toxicological, exposure characteristics, as well as hazardous assessment, and other data items designated to be included in registration by the central competent authority. The central competent authority may, by stages, designate the lists of existing chemical substances subject to standard registration, including the names of the chemical substances, quantity thresholds and deadlines for registration, based on the circumstances of the phase 1 registration of existing chemical substances.
1. Phase I Registration
For an existing chemical substance first manufactured or imported in annual quantity of 100 kilograms or more, a registrant shall, within 6 months from the date of occurrence of the fact, apply for the phase 1 registration. No existing chemical substance shall be manufactured or imported, unless the registration approval is obtained within the specified period. Registration for existing chemical substances, manufactured or imported in annual quantity less than 100 kilograms, may be made in accordance with paragraph 1 voluntary.
2. PECs Registration
In response to the impact of the epidemic, EPA revised and issued the "Regulations for Registration of New Chemical Substances and Existing Chemical Substances", and extended the completion period for standard registration of existing chemical substances to 5 years, which means the deadline is 2024 for the registration code which was approved before 2019. Providing the industry with sufficient buffer preparations time. And if the industry submit most of the items such as material property information, the registrant can obtain the completion code in advance, and then complete the hazard and exposure assessment report within the specified time limit.
However, the review time of EPA is 90 working days from the date of receipt, and the review time can be extended once. In order to avoid missing the deadline, it is recommended that the registrant prepare and apply as early as possible.
Registration types for existing chemical substance registration:
Chemical Substance Annual report
We offer technical consultation and support to comply with the regulations of EPA or MOL and provide long-term maintenance service after approval, such as confidential extension service and regularly annual declared service from April to September every year.
Legal basis:
According to Toxic and Concerned Chemical Substances Control Act, TCCSCA, Chapter 4 Registration and Reporting of Chemical Substances, Article 30. Chemical substances subject to registration must be regularly declared in compliance with the central competent authority's regulations.
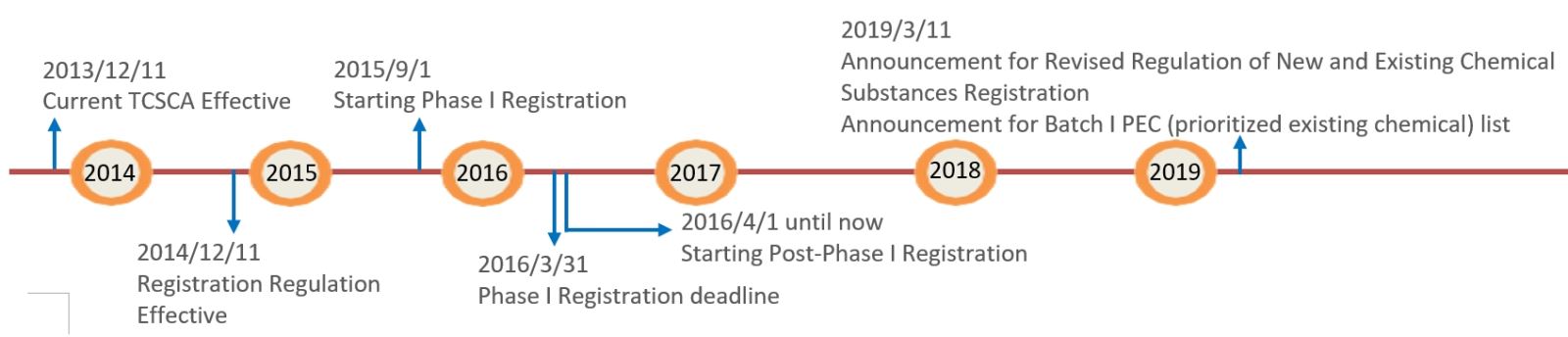
Upcoming registration timeline in the future:
DG Specialty Inc Business Category